Extensive laboratory testing has proven that Antimicrobial Copper continuously kills bacteria*, never wears out, and is safe to use.
In order to obtain the first EPA public health registration for a touch surface material, Antimicrobial Copper alloys had to demonstrate efficacy under rigorous, EPA-approved test protocols. Three test protocols were developed to test Antimicrobial Copper's ability to kill bacteria* that cause infections. A description of the testing is provided below:
Required Testing for Regulatory Approval
The three EPA approved Good Laboratory Practice (GLP) test protocols used to register Antimicrobial Copper with public health claims are:
1. Efficacy as a Sanitizer-which measures viable bacterial count after two hours. For full protocol details, click here.
2. Residual Self-Sanitizing Activity-which measures bacterial count before and after six wet and dry wear cycles during which bacteria are added in a standard wear apparatus (shown as a schematic in Figure 1). For full protocol details, click here.
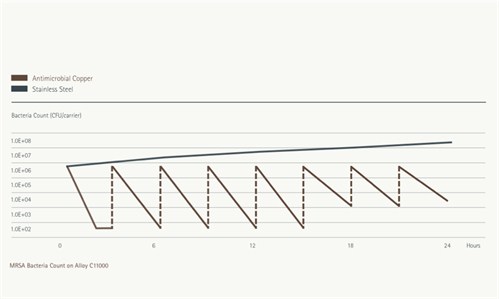
3. Continuous Reduction of Bacterial Contaminants-which measures bacteria after inoculating an alloy surface eight times in a 24-hour period without intermediate cleaning or wiping. For full protocol details, click here.
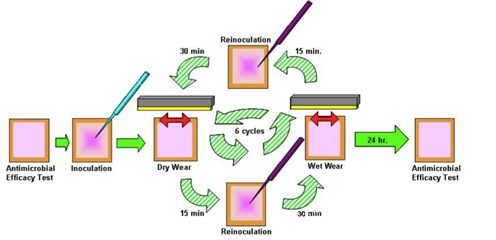
Figure 1: Residual Self-Sanitizing test protocol schematic.
The results of the 216 GLP tests, involving three test protocols, two to three lots of six different alloys, and six bacteria*, are summarized in Table 1. In both the Efficacy as a Sanitizer test and Residual Self-Sanitizing test (wear test), a reduction in live bacteria greater than 99.9% is seen in all seventy two tests when compared to S304. In the Continuous Reduction of Bacterial Contaminants test, a reduction of greater than 99.9% is found in sixty-three out of the seventy-two tests, again when compared to S304. In the remaining nine tests, reductions ranged from 99.3% to 99.9%. In summary, a reduction greater than 99.9% was seen on 207 out of 216 tests. The reduction seen in the remaining nine tests ranged from 99.3% to 99.9%. These results indicate that the antimicrobial response of copper alloys is effective, enduring and reproducible.
Figure 2: Continuous Reduction test results for MRSA on copper alloy C11000 and stainless steel S30400. Each inoculation adds 650,000 CFUs. - Click on graph to enlarge
Table 1: Average Percent Reduction of Bacterial Contamination (Good Laboratory Practice Studies)
|
|
Group |
Alloy |
%Cu |
S.aureus |
E.aerogenes |
MRSA |
P.aeruginosa |
E. coli O157:H7 |
|
Efficacy as a sanitizer |
I |
C110 |
99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
II |
C510 |
94.8 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
III |
C706 |
88.6 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
IV |
C260 |
70 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
V |
C752 |
65 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
VI |
C280 |
60 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
|
|
|
|
|
|
|
|
|
Residual Self Sanitizing |
I |
C110 |
99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
II |
C510 |
94.8 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
III |
C706 |
88.6 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
IV |
C260 |
70 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
V |
C752 |
65 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
VI |
C280 |
60 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
|
|
|
|
|
|
|
|
|
Continuous Reduction |
I |
C110 |
99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
II |
C510 |
94.8 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
III |
C706 |
88.6 |
>99.9 |
>99.9 |
99.9 |
>99.9 |
>99.9 |
|
|
IV |
C260 |
70 |
99.6 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
V |
C752 |
65 |
99.7 |
>99.9 |
>99.9 |
>99.9 |
>99.9 |
|
|
VI |
C280 |
60 |
99.8 |
>99.9 |
99.9 |
>99.9 |
>99.9 |
*Laboratory testing shows that, when cleaned regularly, antimicrobial copper surfaces kill greater than 99.9% of the following bacteria within 2 hours of exposure: MRSA, VRE, Staphylococcus aureus, Enterobacter aerogenes, Pseudomonas aeruginosa, and E. coli O157:H7. Antimicrobial copper surfaces are a supplement to and not a substitute for standard infection control practices and have been shown to reduce microbial contamination, but do not necessarily prevent cross contamination or infections; users must continue to follow all current infection control practices.